Dr. Anthony Porter has long held a passion for innovation and progress.

First earning a B.S. in Engineering from the University of South Carolina, Dr. Porter worked as a nuclear engineer for seven years before choosing to return to school to study medicine. When he finished his Residency in Dermatology at the Medical University of South Carolina in 2002, he held both a doctorate in medicine and membership in Alpha Omega Alpha Medical honor society. After gaining experience in the field, Dr. Porter opened his own practice, Porter Premiere Dermatology and Surgery Center, on Nasa Boulevard in 2010.
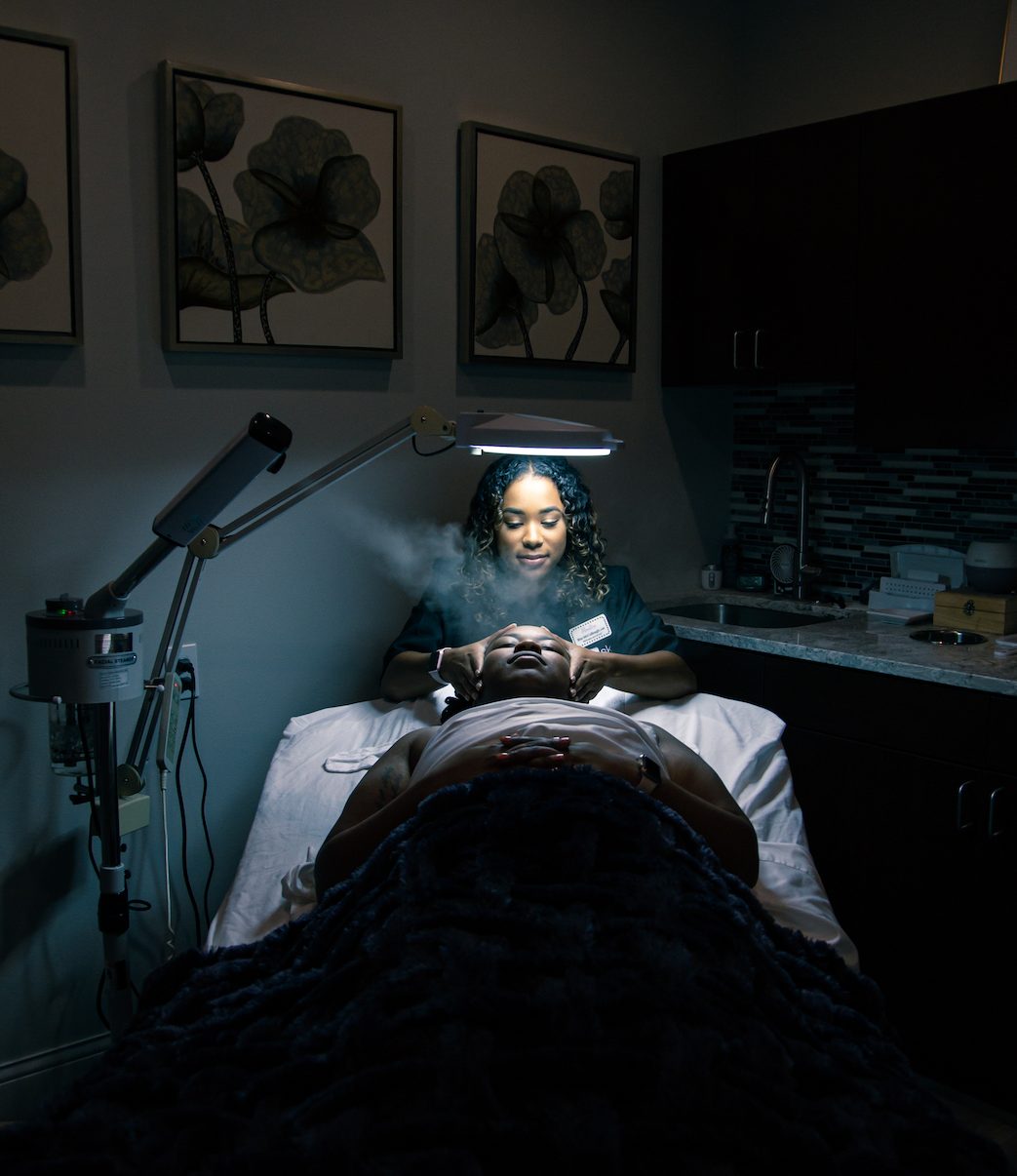
Porter Premiere Dermatology offers general, surgical and, at its brand-new second location on Suntree, cosmetic services. The new office is twofold, with separate waiting and procedure rooms for both sides: One wing is devoted to clinical dermatology and is staffed by experienced medical professionals, while the other wing is Premiere Medical Aesthetics, a spa-style cosmetics center with professional aestheticians. The office also has a nurse practitioner onsite, who does about 95% of the injections for botox and fillers for the whole practice.
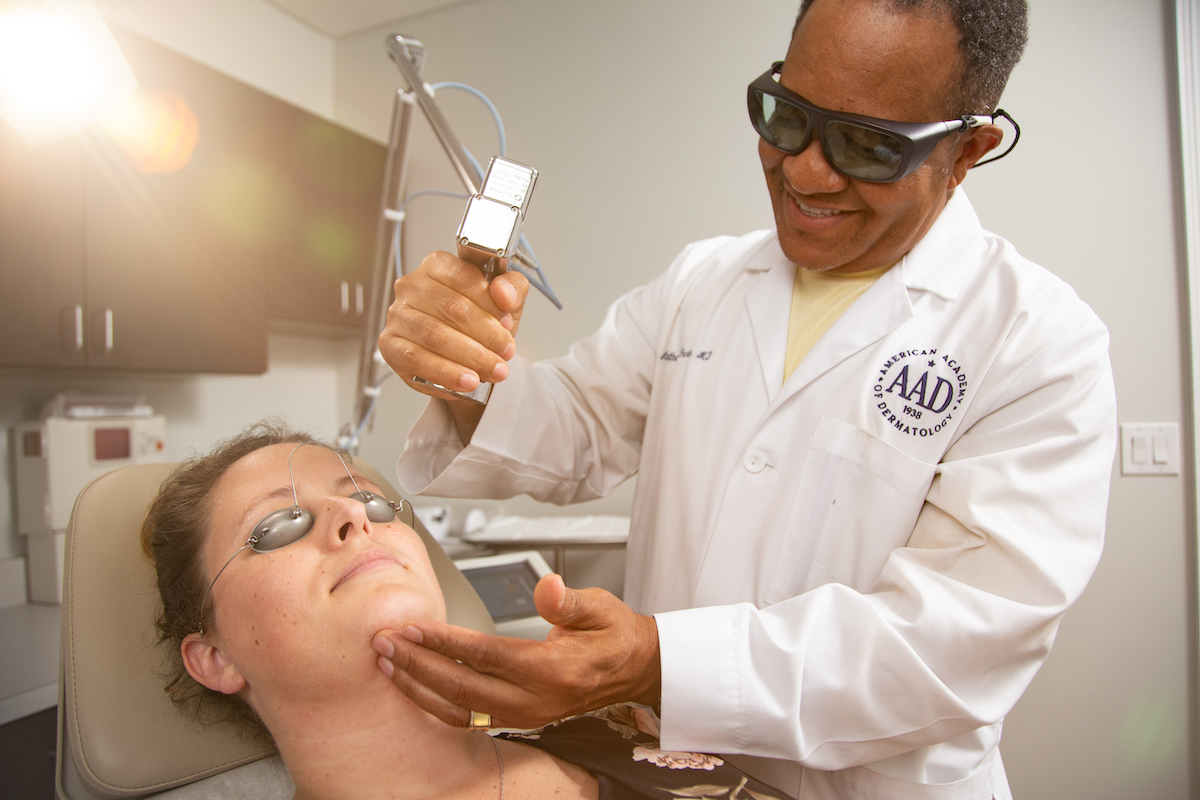
Unlike most dermatology practices, Porter Premiere offers services to anyone regardless of age. You won’t find another practice to offer full-body phototherapy nor another dermatology office with Ultrasound-guided superficial radiotherapy for non-melanoma skin cancers in the area. With this kind of forward-thinking, it’s no surprise that Dr. Porter is an expert on dermatology’s latest development, a life-changing form of treatment known as biologics.
FROM THE DR.
Approximately 3% of the world’s population suffers from psoriasis. Caused by a hyperactive immune system, it is systemic (effecting the full body) and presents as round, red, scaly plaques on the skin and characteristic changes in the nails. One-third of patients with psoriasis eventually develop psoriatic arthritis, which can lead to deformity and destruction of joints. While mild, isolated forms of psoriasis typically respond to topical steroid ointments or creams or phototherapy, moderate to severe cases may require a treatment that affects the whole body. In 2003, a new class of medication called biologics, which are antibodies and proteins engineered to prevent the psoriatic inflammation, were developed to treat more aggressive psoriasis.

Before biologics, systemic treatments for psoriasis involved the use of strong medications like methotrexate and cyclosporine that suppress the immune system. These drugs are effective; however, their use can lead to kidney and liver disease and, due to their immunosuppression, greatly increase a patient’s risk of developing an infection. Biologics provide a more targeted, nuanced approach, inhibiting only portions of the chain of inflammation, called the inflammatory cascade, that causes psoriasis, thereby resulting in more effective, safer treatments with much less immunosuppression. The efficacy of these biologics is remarkable: Most patients achieve clear or almost clear results. Not only do biologics improve the psoriasis of the skin and nails, but they greatly diminish the painful and destructive inflammation that leads to arthritis. The improvement in the quality of life is life-changing, as most patients can achieve these impressive results without the need for creams, ointments or phototherapy.
Currently, 12 FDA-approved biologics for psoriasis are available. Eleven of the 12 are administered by injection, with only one, Infliximab, requiring intravenous infusion. There is no ideal or first-line biologic. Instead, the physician chooses which biologic to use in consultation with the patient, giving consideration to frequency of dosing, patient weight, involvement of joints, body parts affected and drug cost. The biologics currently fit into three main categories based on which specific portion of the immune system inflammatory cascade they target: Tumor Necrosis Factor inhibitors such as Adalimumab (HUMIRA) and Etanercept (ENBREL), Interleukin-17 inhibitors such as Secukinumab (COSENTYX) and Ixekizumab (TALTZ) and Interleukin 23 inhibitors such as Guselkumab (TREMFYA) and Risankizumab (SKYRIZI). We teach patients how to self-administer these products by an injection just under the skin of the abdomen or upper thigh using a small, user-friendly syringe or an auto injector pen. Most of these products are approved for adults 18 years of age and older, but a few are also FDA-approved for teens and adolescents.

The safety profile of these biologics continues to improve as newer agents get approved. Before starting biologics, all patients must be screened for active tuberculosis. In addition, I obtain comprehensive blood tests, including tests for Hepatitis B and C. The patient must also be free of any active infection. Patients with minimally active human immunodeficiency virus (HIV) but otherwise average health have been successfully treated with the IL-23 biologics. The TNF class agents carry a very small risk of organ cancers and skin cancers. Likewise, the IL-23 agents must be used with caution in patients with inflammatory bowel disease. Certolizumab (CIMZIA) is the only agent that has been shown to be safe in pregnancy and breast-feeding. Patients are encouraged to get all required vaccinations prior to starting a biologic, especially as live vaccines cannot be given during biologic therapy. The most commonly used live vaccine on the market is the shingles vaccine, but there is now a new attenuated (inactive) shingles vaccine available for use.
Access to biologics has been a challenge for some patients due to a variety of reasons. An important element is the relatively low number of dermatologists who are willing to prescribe these products. Dermatologists have traditionally been quite cautious about recommending medications that suppress the immune system, and thus have not been very aggressive about prescribing biologics. Fortunately, over the past several years this trend has changed as more dermatologists are recognizing the incredible effectiveness of these products along with the relatively clean safety profile. I have been a long-term advocate for and prescriber of biologic therapy for patients with psoriasis over the past 16 years. Another critical factor is the incredible costs of these drugs. Unfortunately, these products can range in cost from $30,000 – $40,000 per year. Commercial insurers and certain Medicaid providers play a critical role in helping patients gain access to these agents by covering most costs for the patient. The pharmaceutical industry has also helped with access by establishing foundations and co-pay assistance programs that help to expand availability to these valuable medications. Patients over the age of 65 who rely solely on Medicare also have difficulty accessing these drugs due to the exorbitant cost.

Another product for psoriasis that is FDA-approved and worth mentioning is apremilast (OTEZLA). This drug also inhibits certain inflammatory proteins, but it is not immunosuppressive and has therefore gained popularity with patients who may be concerned about suppression of their immune system. Though not considered a biologic, this product, which is administered in tablet form, has delivered reasonably good results in the treatment of psoriasis. The American Academy of Dermatology and the National Psoriasis Foundation are great sources of information about treatments for psoriasis.
Atopic dermatitis (eczema) is another systemic, immune-related disorder that is characterized by inflamed, scaly red patches and plaques on various parts of the skin. The condition can be associated with asthma, seasonal allergies, sinusitis and/or hay fever. Patients can have only a few isolated lesions, or, have almost their entire skin surface affected by this disorder. The lesions are intensely itchy leading to constant picking and scratching of the skin that severely decreases the patient’s quality of life. Most cases are mild and typically respond to lotions, topical steroid creams and antihistamines. However, severe cases of atopic dermatitis require more aggressive treatment. The first biologic developed to target the specific inflammatory process that leads to atopic dermatitis was FDA-approved and launched in 2017. This product, Dupilumab (DUPIXENT) has revolutionized the way I treat atopic dermatitis and has drastically improved the lives of patients suffering from this debilitating condition. This agent modulates the immune system by inhibiting specific inflammatory proteins but does not suppress the whole immune system. DUPIXENT is now approved for patients with atopic dermatitis as young as 12 years old.
The new age of biologics is incredibly exciting as the medical industry continues to develop targeted, safer, more effective therapies. Cosmetic dermatology is an ever-expanding field as patients seek aesthetic procedures to approve their appearance. However, biologics have breathed new life into the field of general dermatology for physicians like me who have a passion for treating inflammatory diseases.

Dr. Anthony Porter
Anthony Porter, M.D., F.A.A.D., received his medical degree and completed a formal dermatology residency at the Medical University of South Carolina. With offices in Melbourne and Suntree, Dr. Porter is board certified in dermatology and has practiced in Melbourne since 2006. For more information, call (321) 308-0659 or visit online at www.porterderm.com.

Heather Motro
In addition to writing and serving as Assistant Managing Editor for Space Coast Magazines, Heather Motro writes the sustainability blog The Blergh, manages social media for the Marine Resources Council and was co-Editor-in-Chief of Holy Trinity High School’s award-winning yearbook, The Tigrium. She is a member of Clemson University Honors College Class of ’24 (go Tigers!).











